WASHINGTON D.C. (WBAP/KLIF News) – The recall of Similac baby formula made by Abbott Nutrition has expanded after a second infant died.
The U.S. Food and Drug Administration posted updated guidelines on its website this week and said investigators are looking into another illness due to a Cronobacter sakazakii infection connected to products from the Sturgis, Michigan facility.
Exposure to the germ can cause blood infections and premature infant death.
The FDA said the investigation includes four reports of the Cronobacter sakazakii infections and one complaint of a Salmonella Newport infection, adding that Cronobacter may have led to the deaths of two infants.
The second child reportedly consumed Abbott Nutrition’s Similac PM 60/40 with a case lot code 27032K800 before being hospitalized with the ultimately fatal infection.
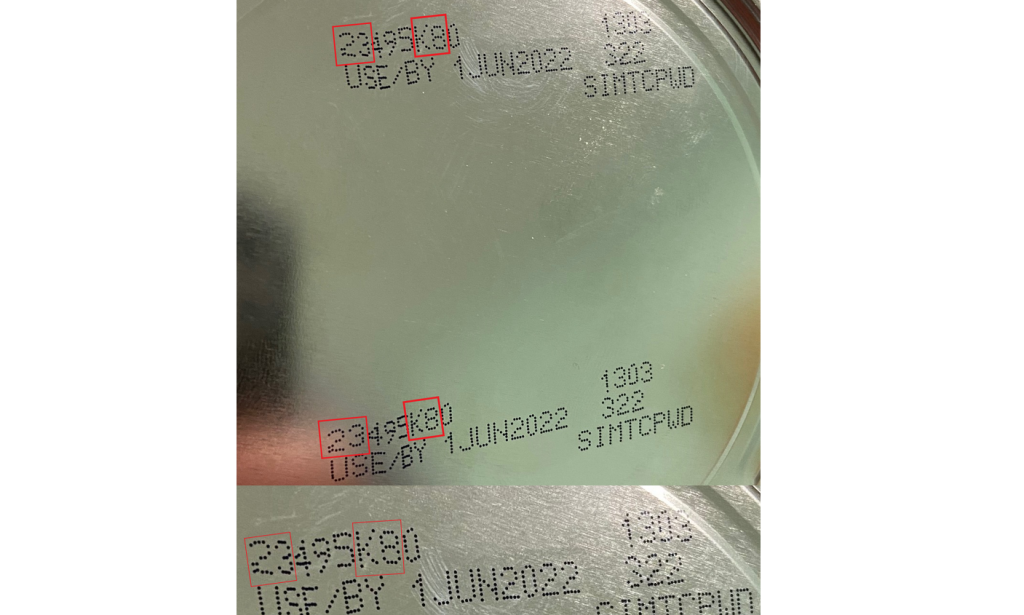
The recalled Similac PM 60/40 products were distributed in the United States and Israel.
The order includes lot code 27032K80 (can) and 27032K800 (case).
This product was not included in last month’s recall of Similac, Alimentum and EleCare formulas.
The FDA said consumers who want to check if their powdered formula is part of the recall can enter the product lot code on the bottom of the package here.
The FDA is working with Abbott Nutrition to safely resume production at the Sturgis facility.
Click here for more information.
Copyright 2021. WBAP/KLIF News. All Rights Reserved.